Patient Support Line 1-800-282-7630, Monday – Friday: 9 AM – 5 PM ET
Proven Survival Results in HR+, HER2- mBC
KISQALI is approved for both pre- and postmenopausal women or in men with HR+, HER2- metastatic breast cancer (mBC). KISQALI has extended lives in multiple clinical trials when taken in combination with a nonsteroidal aromatase inhibitor or fulvestrant. Watch the video below to see why women are asking for KISQALI by name.
Learn more about the difference between progression-free survival and overall survival.
KISQALI + A NONSTEROIDAL AROMATASE INHIBITOR (NSAI) HAS BEEN PROVEN TO HELP POSTMENOPAUSAL WOMEN LIVE A LONGER LIFE
In a clinical trial of 668 women, 334 were treated with KISQALI + an NSAI and 334 women were treated with an NSAI alone. The main result of the study, or primary end point, was progression-free survival (PFS). Overall survival (OS) was an additional result, or secondary end point, of the study.
Proven to help postmenopausal women live longer
Significantly more effective at delaying disease progression than placebo + an NSAI
Live Longer Without Disease Progression
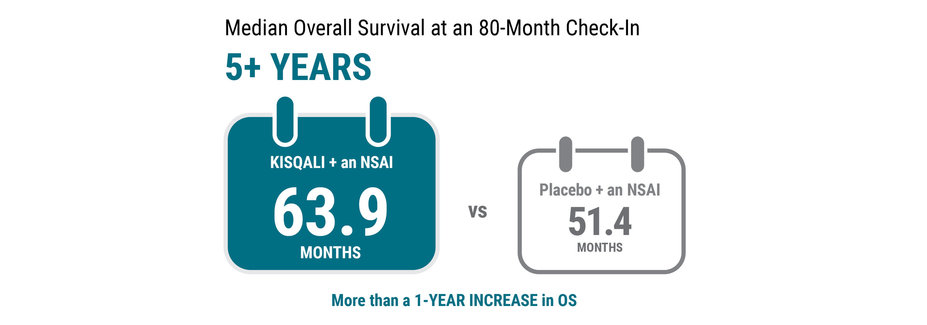
In this clinical trial, KISQALI + an NSAI extended the length of time women were alive from the start of treatment—also called OS.
At an 80-month check‑in, results showed the median OS was 63.9 months for KISQALI + an NSAI vs 51.4 months for placebo + an NSAI.
Median OS is the length of time when half of the women were still alive.
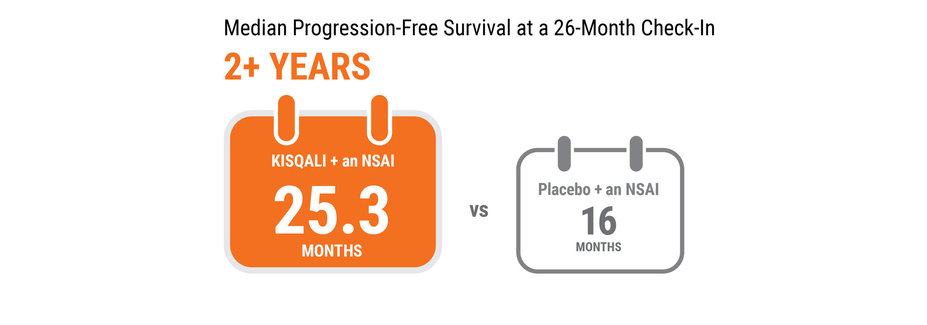
At a 26-month check-in, half the women taking KISQALI + an NSAI still showed no disease progression at 25.3 months vs 16 months for women taking placebo + an NSAI.
Median PFS is the length of time when half of the women had not yet progressed.
KISQALI + AN NSAI + GOSERELIN HAS BEEN PROVEN TO HELP PREMENOPAUSAL WOMEN LIVE A LONGER LIFE
In a clinical trial of 672 women who were premenopausal or perimenopausal when the study started, the median age was 44 years (ranging 25 to 58). In a subgroup analysis of this trial, 248 women were treated with KISQALI + an NSAI (letrozole or anastrozole) + goserelin, and 247 women were treated with an NSAI + goserelin.The main result of the study, or primary end point, was PFS. OS was an additional result, or secondary end point, of the study.
Proven to help premenopausal women live longer
Significantly more effective at delaying disease progression than placebo + an NSAI + goserelin
Proven to Help Premenopausal Women Live Longer Without Disease Progression
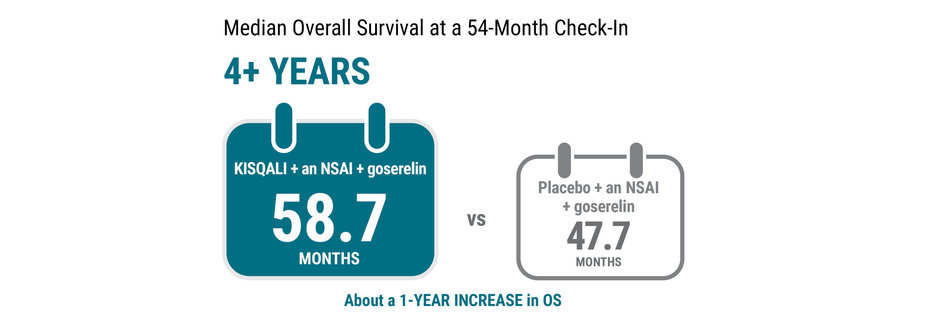
In this clinical trial, KISQALI + an NSAI + goserelin extended the length of time that premenopausal women were alive from the start of treatment—also called OS.
At a 54-month check‑in, the median OS was 58.7 months for KISQALI + an NSAI + goserelin. Median OS was 47.7 months for placebo + an NSAI + goserelin. This 54‑month analysis was not preplanned to detect a false positive or show a difference between treatments.
Median OS is the length of time when half of the women were still alive.
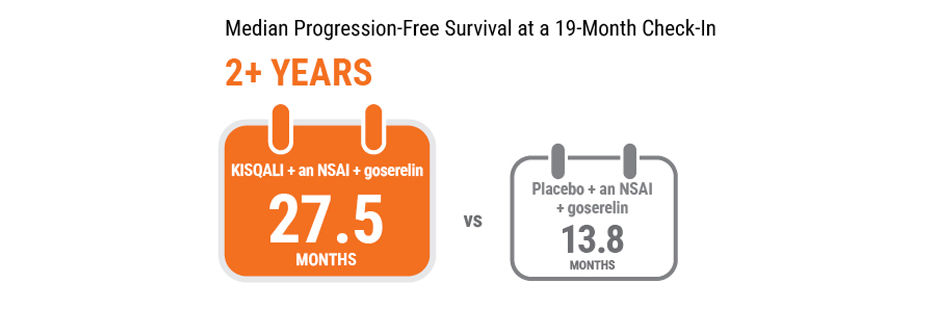
At a 19-month check‑in, half the women taking KISQALI + an NSAI + goserelin still showed no disease progression after 27.5 months vs 13.8 months for women taking placebo + an NSAI + goserelin.
Median PFS is the length of time when half of the women had not yet progressed.
KISQALI is not approved for use with tamoxifen. KISQALI may cause increased risk of heart rhythm problems (QT prolongation) when combined with tamoxifen.
KISQALI + FULVESTRANT HAS BEEN PROVEN TO HELP POSTMENOPAUSAL WOMEN LIVE A LONGER LIFE
In a clinical trial of 726 women, 484 were treated with KISQALI + fulvestrant and 242 women were treated with fulvestrant alone. The main result of the study, or primary end point, was PFS. OS was an additional result, or secondary end point, of the study.
Proven to help postmenopausal women live longer
Significantly more effective at delaying disease progression than placebo + fulvestrant
Proven to Help Women Live a Longer Life Without Disease Progression
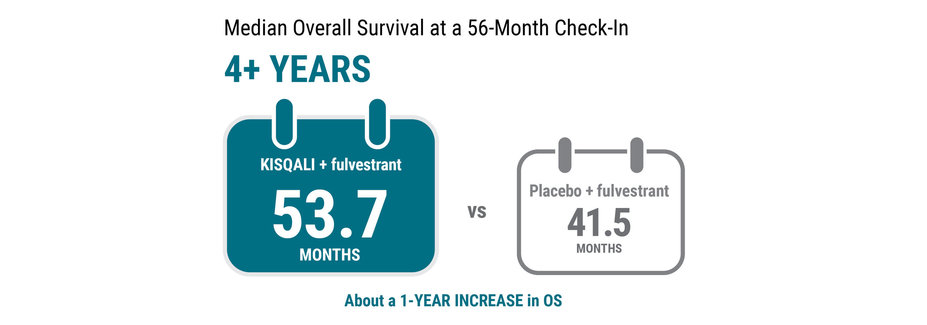
In this clinical trial, KISQALI + fulvestrant extended the length of time women were alive from the start of treatment—also called OS.
At a 56-month check‑in, results showed the median OS was 53.7 months for KISQALI + fulvestrant vs 41.5 months for placebo + fulvestrant. This 56‑month analysis was not preplanned to detect a false positive or show a difference between treatments.
Median OS is the length of time when half of the women were still alive.
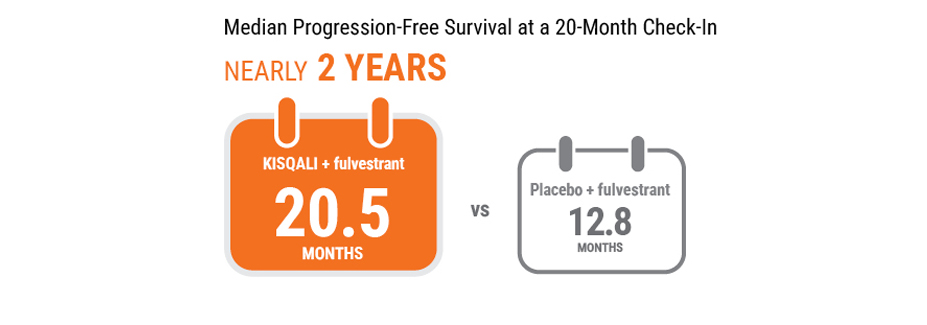
At the 20-month check-in, half the women taking KISQALI + fulvestrant still showed no disease progression after 20.5 months vs 12.8 months for women taking placebo + fulvestrant.
Median PFS is the length of time when half of the women had not yet progressed.